CT abdomen general
Indication/Technique
Please see the X-ray/CT Technique class for additional information about the technique of ‘computer tomography’ (CT).
Abdominal CT scans are generally evaluated in the transversal direction; the patient is seen from the feet upward as it were. The abdomen can also be viewed in the coronal and sagittal directions (fig. 1).
Abdominal CT scans are generally evaluated in the transversal direction; the patient is seen from the feet upward as it were. The abdomen can also be viewed in the coronal and sagittal directions (fig. 1).
Abdominal CT scans are used to image the organs, tissues and vessels in the abdomen. The indication for this examination is very important and is used to decide whether the examination is performed with oral, rectal or intravenous contrast agent. Additionally, when performing a CT scan with intravenous contrast agent, the phase(s) in which the scan is made is also relevant.
Oral contrast agent
Depending on the indication, patients drink a predetermined volume of oral contrast agent prior to the examination. This may be a positive contrast agent (diluted iodinated contrast agent, ‘white’/dense) or negative contrast agent (including water, ‘dark’/hypodense). The contrast agent is in the small intestine during the examination, allowing it to be effectively identified and distinguished from other organs and tissues. Oral contrast agents are particularly valuable in patients with limited intraperitoneal fat (fig. 2).
Rectal contrast agent
Depending on the indication, contrast agent is administered rectally immediately before the examination (fig. 3). Again, this may be a positive or negative contrast agent. One of the primary indications for administering rectal contrast agent is to demonstrate a leak, e.g. in the event of an abnormality following recent intestinal surgery where the distal colon was sutured. The contrast agent then passes through the intestinal lumen. Another example of an indication for administering rectal contrast agent is to demonstrate the presence of a fistula. In the event of a rectovesical fistula, contrast agent will enter the urinary bladder through the fistula. Another example is to demonstrate abscesses in the pelvis minor located between the intestines
ntravenous contrast agent
Firstly, the administration of intravenous (IV) contrast agent allows for effective evaluation of the arteries and veins. The administered contrast agent is transported to the heart through a vein in an arm or leg and is then pumped around through arteries and veins. Additionally, enhancements (blood supply, perfusion) of the abdominal organs can be evaluated. At fixed time intervals after administration the contrast agent, mixing with the blood, will arrive at various sites in the body, which can then be scanned. Scan phases include the arterial, portal venous, nephrogenic and excretion phases (fig. 4).
CT without contrast
This scan is made without the administration of IV contrast agent. A primary indication for abdominal scans without contrast agent is the detection of renal or ureteral stones.
Arterial
This scan is made about 20-30 seconds after the administration of IV contrast agent. The contrast agent is still in the arteries and some organs are starting to absorb the agent. This scan phase is particularly suited for evaluating arteries and detecting hypervascular abnormalities, e.g. hypervascular metastases in the liver (this will be discussed in more detail in the liver lesions section).
Portal venous
This scan is made about 60-80 seconds after the administration of IV contrast agent. This is the most commonly used scan phase. In this phase, the contrast agent is for the most part in the veins. The abdominal organs have absorbed the contrast agent and are ‘enhanced’. This scan phase is generally used to screen for abdominal abnormalities and detect hypovascular liver metastases (this will be discussed in more detail in the liver lesions section).
Nephrogenic
This scan is made about 80-100 seconds after the administration of IV contrast agent. In this phase, both the renal cortex and medulla are homogeneously enhanced. This allows for effective evaluation of the renal parenchyma. This phase is used in particular to evaluate kidney tumors.
Equilibrium/delayed
This scan is made about 6-10 minutes after the administration of IV contrast agent. This phase is also termed the washout or delayed phase. The contrast agent has passed through all the organs and is being excreted by the kidneys. This phase is used frequently to evaluate the urinary tract. In addition, this phase can help characterize liver lesions or detect bile duct tumors.
Many patients require multiple-phase scans, e.g. in abdominal trauma or in liver, pancreas and kidney tumors.
Abdominal CTs generally have multiple slice thicknesses. The slice thickness is commonly shown in the top left corner of the screen, under the patient data. When starting a standard evaluation of an abdominal CT, a slice thickness of 5 mm is recommended. The 1-mm slices are recommended for more detailed analysis of abnormalities or small structures.
Abdominal CTs generally have multiple slice thicknesses. The slice thickness is commonly shown in the top left corner of the screen, under the patient data. When starting a standard evaluation of an abdominal CT, a slice thickness of 5 mm is recommended. The 1-mm slices are recommended for more detailed analysis of abnormalities or small structures.
Normal anatomy
Figure 5 lets you scroll through a normal abdominal CT in the portal venous phase. Unfortunately, not all organs can be discussed in detail in view of their complex anatomy.
A few characteristics of normal anatomy:
A few characteristics of normal anatomy:
- Portal venous phase: the parenchyma of the liver/spleen/pancreas is homogeneously enhanced.
- Intra-abdominal fat has the density of fat (HU -50 to -100; see the X-ray/CT technique course for more information about Hounsfield units); similar to normal subcutaneous fat. If not, there may be ascites or fatty infiltration.
Checklist
Protocol
As discussed under Technique, there are many different ways to perform an abdominal CT. The execution of an abdominal CT is decided on the basis of the indication and question. Because an abdominal CT is not risk-free (think of radiation burden and contrast nephropathy), it is important to do it correctly the first time.
History
Before evaluating an abdominal CT, you should first carefully study the case history. The patient’s medical history is always important. See if previous abdominal CTs have been made, which you could use for comparison. It would be a waste of your time to focus on abnormalities which have been analyzed before.
Additionally, the patient’s general history and clinical status are important when evaluating an abdominal CT, think of (recent) abdominal surgery, radiotherapy, fever/elevated infection parameters.
Additionally, the patient’s general history and clinical status are important when evaluating an abdominal CT, think of (recent) abdominal surgery, radiotherapy, fever/elevated infection parameters.
Quality of the examination
On the basis of the indication and question, a specific scan protocol has been selected. First see if the examination was successful and if the question can be answered. Example: liver metastases are generally invisible on an abdominal CT without contrast agent.
Method
It is recommended to follow a structured method when evaluating a CT. This forces you to examine all organs and reduces the risk of missing anything. Some people prefer to first answer the question and then examine the other organs; others follow a fixed order of organs.
Pathology
Liver
A normal liver enhances homogeneously (irrespective of the scan phase). The liver receives about 80% of its blood through the portal vein (= nutrient-rich blood from the intestines). The remaining 20% is supplied by the hepatic artery.
If focal liver pathology is present, it is important to document its location. This may be crucial to any surgical options. Using the Couinaud classification, the liver is subdivided into eight individually functioning segments. Each segment has its own afferent hepatic artery and portal vein, and efferent hepatic vein and efferent bile ducts (fig
If focal liver pathology is present, it is important to document its location. This may be crucial to any surgical options. Using the Couinaud classification, the liver is subdivided into eight individually functioning segments. Each segment has its own afferent hepatic artery and portal vein, and efferent hepatic vein and efferent bile ducts (fig
Liver cirrhosis is the result of chronic liver disease, causing irreversible damage to the liver tissue. The liver is small and proportions have changed; the left liver lobe and segment 1 are hypertrophic, and the right liver lobe is atrophic. The liver tissue and surface has a nodular aspect (fig. 7). Liver cirrhosis may increase the pressure in the hepatic vessels, giving rise to ‘portal hypertension’. Signs of portal hypertension include collateral formation, splenomegaly and ascites.
Focal abnormalities
As mentioned previously, multiple-phase abdominal CT is performed when a liver lesion is suspected in order to evaluate the enhancement pattern of the lesion. Lesion morphology often presents clues for diagnosis. In order to arrive at a sure diagnosis, however, additional examination is usually required, mostly in the form of a liver MRI.
Most common benign liver lesions
Cyst
Cysts are very common abnormalities in the liver. A liver cyst may vary in size from a few millimeters up to more than 10 cm. Cysts are sharply delineated with a low density (HU < 10). Cysts do not enhance (fig. 9).
Cysts are very common abnormalities in the liver. A liver cyst may vary in size from a few millimeters up to more than 10 cm. Cysts are sharply delineated with a low density (HU < 10). Cysts do not enhance (fig. 9).
Abscess
Abscesses in the liver are usually a complication of an intestinal infection. The bacteria migrate to the liver through the venous system. Patients with a liver abscess are sick, have a fever and elevated infection parameters in the blood. The abscess is usually a cluster of jaggedly delineated hypodensities. The abscess rim may enhance (note: rim enhancement is commonly absent).
Abscesses in the liver are usually a complication of an intestinal infection. The bacteria migrate to the liver through the venous system. Patients with a liver abscess are sick, have a fever and elevated infection parameters in the blood. The abscess is usually a cluster of jaggedly delineated hypodensities. The abscess rim may enhance (note: rim enhancement is commonly absent).
Hemangioma
Hemangiomas are common abnormalities in the liver. A hemangioma can be up to 10 cm in size. Hemangiomas are sharply delineated with a specific enhancement pattern. The arterial phase reveals peripheral, nodular, discontinuous enhancement and the portal venous phase reveals progressive filling. Characteristic of hemangiomas is that the enhancement in each phase corresponds with the ‘blood pool’ (table 2).
Hemangiomas are common abnormalities in the liver. A hemangioma can be up to 10 cm in size. Hemangiomas are sharply delineated with a specific enhancement pattern. The arterial phase reveals peripheral, nodular, discontinuous enhancement and the portal venous phase reveals progressive filling. Characteristic of hemangiomas is that the enhancement in each phase corresponds with the ‘blood pool’ (table 2).
Focal nodular hyperplasia
Focal nodular hyperplasia (FNH) is more common in women than men. FNH arises from liver cells and bile duct cells, and is sharply delineated and hypervascular. Characteristic of FNH is the star-shaped fibrous core in the middle of the tumor, the so-called central scar (table 2). In most FNHs, the fibrous central scar enhances in the equilibrium/delayed phase.
Focal nodular hyperplasia (FNH) is more common in women than men. FNH arises from liver cells and bile duct cells, and is sharply delineated and hypervascular. Characteristic of FNH is the star-shaped fibrous core in the middle of the tumor, the so-called central scar (table 2). In most FNHs, the fibrous central scar enhances in the equilibrium/delayed phase.
Adenoma
Adenomas are particularly common in women aged 20-50 years, but may also occur in men. Oral contraceptive use constitutes a risk factor for developing an adenoma. Large adenomas may bleed or become malignant. Sizes vary markedly: from 1 cm up to more than 20 cm. Adenomas are hypervascular, usually clearly delineated, encapsulated and may contain fat. The bleedings and presence of fat may give the adenoma a heterogeneous aspect (table 2). The enhancement pattern varies. About 20% have enhancement of the (pseudo)capsule in the equilibrium/delayed phase.
Adenomas are particularly common in women aged 20-50 years, but may also occur in men. Oral contraceptive use constitutes a risk factor for developing an adenoma. Large adenomas may bleed or become malignant. Sizes vary markedly: from 1 cm up to more than 20 cm. Adenomas are hypervascular, usually clearly delineated, encapsulated and may contain fat. The bleedings and presence of fat may give the adenoma a heterogeneous aspect (table 2). The enhancement pattern varies. About 20% have enhancement of the (pseudo)capsule in the equilibrium/delayed phase.
Most common malignant liver lesions
Metastasis
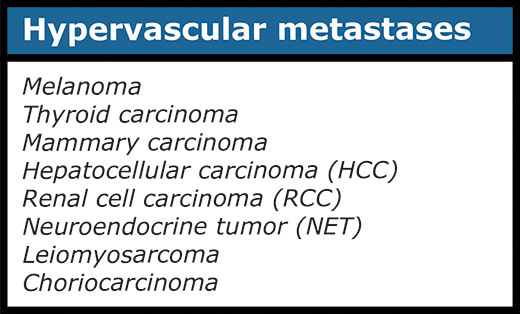
Logically, metastases develop in people with a malignancy, but the primary malignancy may not be known yet. Metastases are vaguely delineated. There are both hypovascular and hypervascular liver metastases (table 6). It is important to realize that hypervascular metastases may be very difficult or impossible to see on a scan in the portal venous phase (fig. 9).
Logically, metastases develop in people with a malignancy, but the primary malignancy may not be known yet. Metastases are vaguely delineated. There are both hypovascular and hypervascular liver metastases (table 6). It is important to realize that hypervascular metastases may be very difficult or impossible to see on a scan in the portal venous phase (fig. 9).
It is therefore important to know beforehand whether a patient is known (or suspected) to have a tumor giving rise to hypervascular metastases. It can then be decided to scan in the arterial phase also. Table 3 summarizes tumors that may give rise to hypervascular metastases.
Hepatocellular cell carcinoma
Hepatocellular cell carcinoma (HCC) is a malignant tumor arising from hepatocytes. HCC is strongly associated with chronic liver diseases such as hepatitis B and C and liver cirrhosis. HCC is more prevalent in non-Western countries than Western countries. HCC is an infiltrative tumor that may proliferate into the veins. Characteristic of HCC is the marked arterial enhancement and the rapid washout of contrast agent in the portal venous and equilibrium/delayed phases (fig. 10)
Hepatocellular cell carcinoma (HCC) is a malignant tumor arising from hepatocytes. HCC is strongly associated with chronic liver diseases such as hepatitis B and C and liver cirrhosis. HCC is more prevalent in non-Western countries than Western countries. HCC is an infiltrative tumor that may proliferate into the veins. Characteristic of HCC is the marked arterial enhancement and the rapid washout of contrast agent in the portal venous and equilibrium/delayed phases (fig. 10)
Cholangiocarcinoma
Cholangiocarcinoma is a bile duct malignancy. The development of cholangiocarcinoma is associated with bile duct cysts and primary sclerosing cholangitis (PSC). The tumor may arise from both the intrahepatic and extrahepatic bile ducts. Characteristic of cholangiocarcinoma is the fibrous nature with capsular retraction and persisting enhancement in the equilibrium/delayed phase and dilation of the proximal bile ducts (table 4).
Cholangiocarcinoma is a bile duct malignancy. The development of cholangiocarcinoma is associated with bile duct cysts and primary sclerosing cholangitis (PSC). The tumor may arise from both the intrahepatic and extrahepatic bile ducts. Characteristic of cholangiocarcinoma is the fibrous nature with capsular retraction and persisting enhancement in the equilibrium/delayed phase and dilation of the proximal bile ducts (table 4).
Gallbladder and bile ducts
Normal undilated intrahepatic bile ducts are invisible on an abdominal CT.
The left and right hepatic duct join to form the common hepatic duct. These in turn join the cystic duct to form the choledochous duct. The choledochous duct eventually joins the pancreatic duct at the level of Vater's papilla, where the bile and pancreatic juice is released into the duodenum (fig. 11).
The choledochous duct is frequently identifiable on CT scans; it should be < 6 mm.
The left and right hepatic duct join to form the common hepatic duct. These in turn join the cystic duct to form the choledochous duct. The choledochous duct eventually joins the pancreatic duct at the level of Vater's papilla, where the bile and pancreatic juice is released into the duodenum (fig. 11).
The choledochous duct is frequently identifiable on CT scans; it should be < 6 mm.
Bile stones
Bile stones may develop in the gallbladder (cholecystolithiasis) and may migrate to the bile ducts (choledocholithiasis). Bile stones are generally invisible on CT scans. Ultrasound is most suited to identify bile stones. However, the consequences of an obstructive bile stone can be seen on CT. The obstruction prevents the bile from passing. With an obstructive stone in the gallbladder, the gallbladder becomes enlarged (hydropic). With an obstructive stone in the bile ducts, the bile ducts are dilated; the intrahepatic bile ducts are dilated when their diameter exceeds 2 mm, the choledochous duct is dilated when its diameter exceeds 6 mm (fig. 12).
Bile stones may develop in the gallbladder (cholecystolithiasis) and may migrate to the bile ducts (choledocholithiasis). Bile stones are generally invisible on CT scans. Ultrasound is most suited to identify bile stones. However, the consequences of an obstructive bile stone can be seen on CT. The obstruction prevents the bile from passing. With an obstructive stone in the gallbladder, the gallbladder becomes enlarged (hydropic). With an obstructive stone in the bile ducts, the bile ducts are dilated; the intrahepatic bile ducts are dilated when their diameter exceeds 2 mm, the choledochous duct is dilated when its diameter exceeds 6 mm (fig. 12).
Cholecystitis
A complication of bile stones is an infected gallbladder or cholecystitis. Cholecystitis rarely occurs in the absence of bile stones. Ultrasound is also best suited to diagnose cholecystitis. Ultrasound improves the visibility of the bile stones, and gallbladder compressibility can be evaluated (dynamic examination). Absent compressibility constitutes a key characteristic of cholecystitis (see abdominal ultrasound class). Other characteristics of cholecystitis on CT include gallbladder wall thickening (fig. 13/14) and infiltration of the fat surrounding the gallbladder. A common complication of cholecystitis is gallbladder perforation, where bile leaks into the abdominal cavity (biloma).
A complication of bile stones is an infected gallbladder or cholecystitis. Cholecystitis rarely occurs in the absence of bile stones. Ultrasound is also best suited to diagnose cholecystitis. Ultrasound improves the visibility of the bile stones, and gallbladder compressibility can be evaluated (dynamic examination). Absent compressibility constitutes a key characteristic of cholecystitis (see abdominal ultrasound class). Other characteristics of cholecystitis on CT include gallbladder wall thickening (fig. 13/14) and infiltration of the fat surrounding the gallbladder. A common complication of cholecystitis is gallbladder perforation, where bile leaks into the abdominal cavity (biloma).
Pancreas
The pancreatic drainage system is variable. Many people have one pancreatic duct which drains into Vater's papilla. Some people have an accessory pancreatic duct, also known as Santorini’s duct (anatomic variation). The accessory pancreatic duct drains into the minor papilla (fig. 15). Other anatomic variations, such as the pancreatic divisum, will not be discussed in this course.
The patient can be asked to drink extra water (about 400 mL) immediately prior to the examination to ensure optimal evaluation of the pancreas. This will cause the stomach and duodenum to distend and help to distinguish the peripancreatic structures.
The pancreas is located in the retroperitoneal cavity and is subdivided into three sections: the head, the body and the tail. A markedly atrophic pancreas can be difficult to recognize. Tips for finding the pancreas: look for the spleen; the pancreatic tail is located close to the spleen. Starting at the tail, follow the pancreas (ventral of the lienal vein) to the head (fig. 16).
The pancreas is located in the retroperitoneal cavity and is subdivided into three sections: the head, the body and the tail. A markedly atrophic pancreas can be difficult to recognize. Tips for finding the pancreas: look for the spleen; the pancreatic tail is located close to the spleen. Starting at the tail, follow the pancreas (ventral of the lienal vein) to the head (fig. 16).
Figure 16. Normal anatomy of the pancreas.
Acute pancreatitis
Pancreatitis is associated with bile stones and alcohol consumption. Autoimmune pancreatitis may also develop. When the pancreas is infected, the pancreatic enzymes damage the parenchyma. Depending on the severity of the infection, infiltrative and necrotizing pancreatitis can be distinguished. Infiltrative pancreatitis is characterized by swelling of the parenchyma and infiltration of the peripancreatic fat (fig. 17/18). In necrotizing pancreatitis, there is necrosis of the parenchyma and/or the peripancreatic fat. Necrotic parenchyma enhances to a lesser degree than healthy parenchyma. Necrosis of the peripancreatic fat gives rise to peripancreatic fluid accumulation
Pancreatitis is associated with bile stones and alcohol consumption. Autoimmune pancreatitis may also develop. When the pancreas is infected, the pancreatic enzymes damage the parenchyma. Depending on the severity of the infection, infiltrative and necrotizing pancreatitis can be distinguished. Infiltrative pancreatitis is characterized by swelling of the parenchyma and infiltration of the peripancreatic fat (fig. 17/18). In necrotizing pancreatitis, there is necrosis of the parenchyma and/or the peripancreatic fat. Necrotic parenchyma enhances to a lesser degree than healthy parenchyma. Necrosis of the peripancreatic fat gives rise to peripancreatic fluid accumulation
Important:
Pancreatitis is usually a clinical diagnosis. It is important to identify necrotizing pancreatitis. This diagnosis cannot be made until symptoms have been present for more than 72 hours. Performing a CT before this time is therefore ineffective, except when the diagnosis is unclear.
Pancreatitis is usually a clinical diagnosis. It is important to identify necrotizing pancreatitis. This diagnosis cannot be made until symptoms have been present for more than 72 hours. Performing a CT before this time is therefore ineffective, except when the diagnosis is unclear.
Pancreatic tumors
Solid pancreatic abnormalities
Adenocarcinoma
Adenocarcinoma is the most common pancreatic malignancy. In the diagnostic workup, surgical options are usually limited by involvement of surrounding vessels (superior mesenteric vein, portal vein, superior mesenteric artery, hepatic artery, celiac trunk) or metastases. Pancreatic adenocarcinoma is a hypovascular tumor, usually arising in the pancreatic head. Depending on its size, the tumor compresses the pancreatic duct and choledochous duct, causing dilation: the double duct sign (fig. 19). The pancreatic tail is frequently atrophied. Preferential sites of metastasis formation include the lymph glands, liver, lungs and peritoneum
Adenocarcinoma is the most common pancreatic malignancy. In the diagnostic workup, surgical options are usually limited by involvement of surrounding vessels (superior mesenteric vein, portal vein, superior mesenteric artery, hepatic artery, celiac trunk) or metastases. Pancreatic adenocarcinoma is a hypovascular tumor, usually arising in the pancreatic head. Depending on its size, the tumor compresses the pancreatic duct and choledochous duct, causing dilation: the double duct sign (fig. 19). The pancreatic tail is frequently atrophied. Preferential sites of metastasis formation include the lymph glands, liver, lungs and peritoneum
Figure 19. Scanned in the arterial phase. Pancreatic head tumor with dilation of the choledochous duct and pancreatic duct, consistent with double duct sign.
Neuroendocrine tumor
Neuroendocrine tumors (including insulinoma, gastrinoma) are usually solid, hypervascular pancreatic tumors. These tumors are visible in the arterial phase in particular.
Neuroendocrine tumors (including insulinoma, gastrinoma) are usually solid, hypervascular pancreatic tumors. These tumors are visible in the arterial phase in particular.
Cystic pancreatic neoplasms
- Pseudocyst: a common benign abnormality is the pseudocyst. Pseudocysts are residual abnormalities after pancreatitis or abdominal trauma. The cyst is unilocular and has no solid components (fig. 20).
- Serous cystadenoma: occurs mostly in middle-aged/elderly women (> 60 years) and is a benign pancreatic lesion. Serous cystadenomas consist of multiple small cysts a few millimeters in diameter, filled with serous fluid (honeycomb-like), and are located predominantly in the pancreatic head. The lesion may contain a (calcified) central scar.
- Mucinous cystadenoma: occurs mostly in women aged 40-50 years. Mucinous cystadenomas consist of one or multiple larger, mucin-filled cysts and may contain parietal calcifications. The lesion may transform to become malignant and develops mostly in the pancreatic body & pancreatic tail.
- Intraductal papillary mucinous neoplasm (IPMN): originates from the pancreatic duct. IPMN may arise from the main branch or a side branch or both (mixed-type). IPMN is a premalignant abnormality; the main-branch type has the highest risk of becoming malignant.
Spleen
Because of its specific vascularization, the spleen enhances inhomogeneously/with stripes in the arterial phase; this is a normal finding. However, the spleen should enhance homogeneously in the portal venous phase (fig.- Splenomegaly
The size of the spleen is measured in the coronal direction, from cranial to caudal. In adults, the normal cranial-caudal size of the spleen is under 12-13 cm.Infarction
As mentioned previously, the spleen enhances inhomogeneously in the arterial phase. The spleen should enhance homogeneously in the portal venous phase. If the spleen does not enhance homogeneously in the portal venous phase, this may be caused by an infarction. A splenic infarction is characterized by a wedge-shaped, hypodense area (fig. 23). -
Splenic trauma
Traumatic splenic damage may result from both blunt and sharp abdominal trauma. As mentioned previously, a multiple-phase abdominal CT is performed when intra-abdominal injury is suspected. ‘Contrast blush’ is visible in the arterial phase in the event of an active bleeding. Splenic laceration is characterized by a linear hypodensity in the spleen in the portal venous phase (fig. 24). There is also free fluid in the abdominal cavity as a result of the bleeding.





























No comments:
Post a Comment